The Role of AI in Advancing Precision Medicine: Forget generic treatments; the future of healthcare is hyper-personalized. Imagine a world where diseases are diagnosed earlier, treatments are tailored to your unique genetic makeup, and even drug discovery is sped up dramatically. That’s the promise of AI in precision medicine, a field poised to revolutionize how we approach illness and wellness. This isn’t just science fiction; it’s happening now, with AI algorithms analyzing medical images, predicting disease risk, and personalizing therapies in ways previously unimaginable.
From analyzing complex genomic data to designing smarter clinical trials, AI is transforming every aspect of healthcare. We’ll delve into how AI-powered tools are improving diagnostics, creating personalized treatment plans, and accelerating the development of new drugs. But it’s not all sunshine and rainbows; we’ll also explore the ethical considerations and potential pitfalls of this rapidly evolving technology, ensuring a balanced and informed perspective.
AI-Driven Diagnostics and Disease Prediction

Source: substackcdn.com
The integration of artificial intelligence (AI) into healthcare is revolutionizing how we diagnose and predict diseases. AI’s ability to process vast amounts of data with incredible speed and accuracy is transforming medical practice, leading to earlier detection, more personalized treatments, and ultimately, better patient outcomes. This section delves into the specific applications of AI in diagnostics and predictive modeling.
AI Image Analysis for Disease Detection
AI algorithms, particularly deep learning models, are proving remarkably adept at analyzing medical images like X-rays, CT scans, and MRIs. These algorithms are trained on massive datasets of labeled images, learning to identify subtle patterns and anomalies that might be missed by the human eye. This allows for faster and more accurate diagnosis, leading to quicker interventions and potentially improved survival rates. For example, AI can detect minute cancerous growths in mammograms far earlier than a human radiologist might, significantly improving the chances of successful treatment.
Accuracy Comparison of AI and Traditional Methods
The following table compares the accuracy of AI-based diagnostic tools with traditional methods for three different diseases. While these figures represent averages from various studies and can vary depending on the specific AI model and dataset used, they highlight the potential of AI to enhance diagnostic accuracy.
| Disease | Traditional Method Accuracy (%) | AI-Based Method Accuracy (%) | Source/Notes |
|---|---|---|---|
| Breast Cancer Detection (Mammography) | 85-90 | 92-95 | Studies published in Radiology and related journals; accuracy varies depending on the AI model and image quality. |
| Diabetic Retinopathy Detection (Fundus Images) | 70-80 | 85-90 | Studies from the American Academy of Ophthalmology; accuracy improves with larger datasets used for training. |
| Lung Cancer Detection (CT Scans) | 80-85 | 88-92 | Studies published in the Journal of Thoracic Oncology; performance heavily reliant on image quality and AI model training. |
Machine Learning for Disease Risk Prediction
Machine learning algorithms excel at identifying patterns in complex datasets to predict the likelihood of an individual developing a specific disease. By analyzing a patient’s genetic information, lifestyle factors (diet, exercise, smoking), medical history, and other relevant data, these algorithms can generate personalized risk scores. This allows for proactive interventions, such as lifestyle changes or preventative screenings, to reduce the risk of disease onset.
Examples of AI models used for risk prediction include: polygenic risk scores (PRS) which combine the effects of multiple genes to predict disease risk, and Cox proportional hazards models which estimate the probability of an event (like disease onset) occurring over time. However, limitations exist. The accuracy of predictions depends heavily on the quality and completeness of the input data. Furthermore, over-reliance on risk scores without considering clinical judgment can lead to misinterpretations and inappropriate interventions. For instance, a high risk score might not necessarily translate to immediate disease development, and a low risk score doesn’t guarantee freedom from the disease.
Development and Validation of an AI-Based Diagnostic Tool for Diabetic Retinopathy
The development and validation of an AI-based diagnostic tool for diabetic retinopathy typically involves the following steps:
- Data Acquisition and Preprocessing: Gathering a large and diverse dataset of fundus images, including both healthy and diseased eyes, with accurate labels. This involves careful image cleaning, standardization, and augmentation to improve model robustness.
- Model Development and Training: Selecting an appropriate deep learning architecture (e.g., convolutional neural network) and training it on the prepared dataset. This involves optimizing model parameters to achieve high accuracy and generalization performance.
- Model Evaluation and Validation: Rigorously evaluating the model’s performance using various metrics (e.g., sensitivity, specificity, AUC) on independent test datasets. This ensures the model’s accuracy and generalizability to unseen data.
- Clinical Validation: Conducting clinical trials to compare the performance of the AI tool with that of human experts. This involves assessing the tool’s diagnostic accuracy, efficiency, and impact on clinical decision-making.
- Regulatory Approval and Deployment: Obtaining necessary regulatory approvals (e.g., FDA clearance) before deploying the tool in clinical practice. This ensures the safety and effectiveness of the AI tool.
Personalized Treatment Strategies
The era of one-size-fits-all medicine is fading. Precision medicine, powered by artificial intelligence, is ushering in a new age of personalized treatment strategies, tailoring therapies to individual patients based on their unique genetic makeup, lifestyle, and disease characteristics. This approach promises to improve treatment outcomes, reduce side effects, and ultimately, save lives.
AI’s ability to analyze vast datasets allows for the identification of subtle patterns and correlations that would be impossible for humans to detect manually. This capability is revolutionizing how we approach various diseases, particularly cancer and chronic conditions like diabetes and heart disease.
AI’s role in precision medicine is revolutionizing healthcare, tailoring treatments to individual needs based on genetic data and lifestyle factors. This hyper-personalization mirrors the efficiency gains seen in other sectors, like the smart city revolution, where interconnected devices optimize resource use; for instance, check out how How IoT is Improving Energy Efficiency in Smart Cities demonstrates the power of data-driven optimization.
Ultimately, both fields showcase the potential of intelligent systems to create more efficient and effective solutions.
AI in Personalizing Cancer Treatment
AI is transforming cancer treatment by analyzing a wealth of data – from tumor genomics and imaging to patient history and lifestyle factors – to create highly individualized treatment plans. This personalized approach allows oncologists to select the most effective therapies, optimize treatment schedules, and predict potential treatment responses and side effects. For instance, AI algorithms can analyze genomic data to identify specific mutations driving tumor growth, guiding the selection of targeted therapies. Similarly, AI can analyze medical images to identify tumor characteristics, helping to determine the optimal treatment strategy, be it surgery, radiation, or chemotherapy. The integration of these diverse data points provides a comprehensive picture of the patient’s unique cancer profile, enabling more precise and effective treatment.
| AI Approach | Data Used | Application | Example |
|---|---|---|---|
| Genomic Profiling | Tumor DNA sequencing, gene expression data | Identifying driver mutations, predicting drug response | Using AI to identify patients who will respond to immunotherapy based on their tumor’s mutational load. |
| Radiomics | Medical images (CT, MRI, PET) | Quantifying tumor characteristics, predicting prognosis and treatment response | AI analysis of CT scans to predict the likelihood of recurrence after surgery. |
| Multi-omics Integration | Genomic, transcriptomic, proteomic, and clinical data | Developing comprehensive patient profiles, predicting treatment outcomes | Combining genomic data with patient history and lifestyle factors to personalize chemotherapy regimens. |
| Machine Learning for Treatment Optimization | Patient data, treatment response, clinical outcomes | Optimizing treatment schedules and dosages, predicting treatment efficacy | Using reinforcement learning to dynamically adjust radiation dosage based on tumor response. |
AI in Drug Discovery and Development
AI is accelerating the drug discovery and development process, significantly reducing the time and cost associated with bringing new therapies to market. AI algorithms can analyze massive datasets of chemical compounds, biological pathways, and clinical trial data to identify potential drug targets, predict drug efficacy, and assess potential toxicity. For example, AI has been instrumental in identifying novel drug candidates for various cancers and neurodegenerative diseases. One notable success is the use of AI to identify molecules with potential antiviral activity against COVID-19, drastically shortening the time it took to develop vaccines and therapeutics. This ability to predict drug efficacy and toxicity early in the development process helps to minimize the risk of failure and accelerate the delivery of life-saving medications.
AI in Personalizing Treatment for Chronic Diseases
AI is also transforming the management of chronic diseases like diabetes and heart disease. Different AI-based approaches are employed to personalize treatment depending on the specific disease and individual patient factors.
The following points highlight some key differences in AI-based personalization strategies for chronic diseases:
- Diabetes: AI algorithms analyze continuous glucose monitoring (CGM) data, lifestyle factors (diet, exercise), and genetic predisposition to predict insulin requirements and optimize insulin delivery systems, leading to better glucose control and reduced complications. AI-powered apps can provide personalized recommendations for diet and exercise based on individual needs and preferences.
- Heart Disease: AI algorithms analyze electrocardiograms (ECGs), echocardiograms, and other cardiac data to identify high-risk individuals, predict cardiovascular events, and personalize treatment strategies, such as medication regimens or lifestyle modifications. AI can also help to optimize the timing and type of interventions like stenting or bypass surgery based on individual patient characteristics.
AI in Genomics and Proteomics
The sheer volume of data generated by genomics and proteomics research presents an unprecedented challenge and opportunity. AI, with its capacity to process and analyze massive datasets, is rapidly becoming an indispensable tool in unlocking the secrets held within our genes and proteins, paving the way for truly personalized medicine. This section delves into the specific applications of AI in these crucial fields.
AI-Powered Genomic Data Analysis for Disease Identification
An AI-powered system for analyzing large genomic datasets would leverage machine learning algorithms, specifically deep learning approaches like convolutional neural networks (CNNs) and recurrent neural networks (RNNs), to identify patterns and correlations indicative of specific diseases. The data would be structured using a graph database, capable of handling complex relationships between genes, variations, and phenotypes. CNNs are particularly well-suited for analyzing genomic sequences, identifying subtle variations and mutations that might be missed by traditional methods. RNNs excel at handling sequential data, such as gene expression time series, revealing dynamic changes associated with disease progression. The system would incorporate various data preprocessing steps, including quality control, normalization, and feature selection, to ensure the accuracy and reliability of the analysis. Feature selection algorithms, such as recursive feature elimination, would help to identify the most relevant genetic markers associated with specific diseases. The system’s output would be a ranked list of genetic markers and their associated probabilities of disease development.
AI-Driven Pharmacogenomics: Predicting Drug Response
AI can predict individual patient responses to medications based on their genomic profiles. This field, known as pharmacogenomics, uses machine learning algorithms to analyze the relationship between an individual’s genetic makeup and their response to drugs. For instance, algorithms like support vector machines (SVMs) and random forests can be trained on datasets containing genomic information and drug response data to predict whether a patient will experience a positive or adverse reaction to a particular medication. A successful example is the use of AI to predict the efficacy of certain cancer therapies based on a patient’s tumor genomic profile. By analyzing the genetic mutations within the tumor, AI algorithms can predict the likelihood of success for targeted therapies, allowing oncologists to tailor treatment plans accordingly. This personalized approach minimizes side effects and maximizes treatment effectiveness.
AI in Proteomics for Biomarker Discovery
AI can analyze proteomic data to identify novel biomarkers for early disease detection. This involves using machine learning algorithms to identify patterns and correlations in complex proteomic datasets, such as those obtained through mass spectrometry. Algorithms like principal component analysis (PCA) and t-distributed stochastic neighbor embedding (t-SNE) can be used for dimensionality reduction, simplifying the analysis of high-dimensional proteomic data. Other algorithms, such as random forests and gradient boosting machines, can be used to build predictive models for disease diagnosis based on the identified protein biomarkers.
| Benefit | Challenge | Example | Mitigation Strategy |
|---|---|---|---|
| High-throughput analysis of complex datasets | Data heterogeneity and noise | Rapid identification of potential Alzheimer’s biomarkers from mass spectrometry data | Robust data preprocessing and advanced noise reduction techniques |
| Discovery of novel biomarkers | Lack of labeled data for training | Identification of novel cancer biomarkers using unsupervised learning methods | Transfer learning from related datasets or use of synthetic data augmentation |
| Improved diagnostic accuracy | Interpretability of complex models | Development of AI-based diagnostic tools for early detection of various diseases | Use of explainable AI (XAI) techniques to improve model transparency |
| Personalized medicine | High computational cost | Development of personalized treatment strategies based on individual proteomic profiles | Use of cloud computing resources and optimized algorithms |
Ethical and Societal Implications
The transformative potential of AI in precision medicine is undeniable, but its implementation isn’t without its ethical and societal hurdles. Balancing the benefits with the potential risks requires careful consideration of several key areas, including data privacy, algorithmic bias, and equitable access to this groundbreaking technology. Ignoring these concerns could lead to unintended consequences, hindering the widespread adoption and ultimately undermining the promise of precision medicine.
The integration of AI into healthcare raises complex ethical dilemmas that demand proactive and thoughtful solutions. Failure to address these concerns head-on risks exacerbating existing health disparities and eroding public trust in this rapidly evolving field.
Data Privacy and Security
The use of AI in precision medicine relies heavily on vast amounts of sensitive patient data, including genomic information, medical history, and lifestyle choices. Protecting this data from unauthorized access and misuse is paramount. Breaches can have devastating consequences, leading to identity theft, discrimination, and erosion of patient trust.
- Robust data encryption and anonymization techniques are crucial to safeguard patient privacy.
- Strict adherence to data governance frameworks, such as HIPAA in the US and GDPR in Europe, is essential.
- Transparent data usage policies and informed consent procedures are necessary to build and maintain patient trust.
Algorithmic Bias and Fairness, The Role of AI in Advancing Precision Medicine
AI algorithms are trained on data, and if that data reflects existing societal biases, the algorithms will perpetuate and even amplify those biases. This can lead to inaccurate diagnoses, inappropriate treatment recommendations, and health disparities across different demographic groups. Addressing algorithmic bias requires careful attention to data quality and algorithm design.
- Careful curation of training datasets to ensure representation of diverse populations is crucial to mitigate bias.
- Regular audits and evaluations of algorithms for fairness and accuracy are necessary to identify and correct biases.
- Developing methods for explaining and interpreting AI decisions can increase transparency and accountability.
Access and Equity
The cost of developing and deploying AI-based tools in healthcare can be substantial, potentially creating a two-tiered system where access is limited to those who can afford it. This could exacerbate existing health inequalities, leaving vulnerable populations behind. Ensuring equitable access to the benefits of AI in precision medicine is a critical ethical imperative.
- Strategies for reducing the cost of AI-based tools are needed to make them accessible to a wider range of patients.
- Public-private partnerships can play a vital role in funding and deploying AI solutions in underserved communities.
- Policies that promote equitable access to AI-driven precision medicine are crucial to achieving health equity.
Regulatory Challenges and Policy Considerations
The rapid advancement of AI in healthcare necessitates a robust regulatory framework to ensure safety, efficacy, and ethical use. This framework needs to balance innovation with patient protection, addressing issues of data privacy, algorithm transparency, and liability.
- The FDA in the US has established guidelines for the development and approval of AI-based medical devices, emphasizing rigorous testing and validation.
- The EU’s GDPR provides a comprehensive framework for data protection, impacting the use of patient data in AI applications.
- Ongoing dialogue and collaboration between regulators, researchers, and healthcare providers are essential to develop adaptable and effective policies.
Infographic Description: AI in Precision Medicine: A Double-Edged Sword
The infographic would be visually divided into two halves, one depicting benefits and the other risks. The benefits side would showcase vibrant imagery of personalized treatment plans, improved diagnostic accuracy (illustrated perhaps with a magnified image of cells highlighting a specific anomaly accurately detected by AI), and reduced healthcare costs through preventative measures. Text would highlight phrases like “Targeted Therapies,” “Early Disease Detection,” and “Improved Patient Outcomes.” The risks side would use a more muted color palette, showing imagery representing data breaches (a cracked computer screen), algorithmic bias (a skewed graph), and unequal access (a stark contrast between a well-equipped hospital and a resource-poor clinic). Text would include warnings like “Data Privacy Concerns,” “Algorithmic Bias,” and “Health Disparities.” A central section would emphasize the importance of ethical guidelines and responsible AI development, acting as a bridge between the two sides, highlighting the necessity of responsible innovation for maximizing benefits and mitigating risks.
AI-Powered Clinical Trials and Research
Source: nextgeninvent.com
The integration of artificial intelligence (AI) is revolutionizing clinical trials, offering the potential to significantly accelerate the development of new treatments and improve the efficiency of research processes. AI’s ability to analyze vast datasets, identify complex patterns, and predict outcomes is transforming how we design, conduct, and interpret clinical trials. This leads to faster development of life-saving medications and therapies.
AI-Driven Optimization of Clinical Trials
The design and execution of clinical trials can be significantly enhanced through AI. By streamlining various stages of the trial process, AI can lead to faster results and more efficient resource allocation.
Methods for Designing AI-Powered Clinical Trials
AI offers several key advantages in optimizing clinical trials. These methods improve recruitment, data collection, and ultimately, speed up the development of new treatments.
- AI-Powered Patient Recruitment: AI algorithms can analyze patient data from electronic health records (EHRs) and other sources to identify individuals who meet the inclusion criteria for specific clinical trials. This targeted approach improves recruitment rates and reduces the time spent searching for eligible participants. For example, an AI system could identify patients with specific genetic markers or clinical characteristics associated with a particular disease, ensuring a more homogenous and representative trial population.
- Intelligent Data Collection: AI-powered wearable sensors and mobile applications can collect real-time data on patients’ physiological parameters, activity levels, and other relevant factors. This continuous monitoring provides a richer dataset than traditional methods, enabling researchers to gain a more comprehensive understanding of the disease and treatment response. Imagine a trial for a new heart medication where AI analyzes data from wearable heart rate monitors, providing insights into individual patient responses not previously possible.
- Predictive Modeling for Trial Design: AI can be used to develop predictive models that optimize trial design parameters, such as sample size, duration, and endpoint selection. These models can help to minimize the risk of failure and maximize the chances of success. For instance, an AI model might predict the optimal dosage of a drug based on patient characteristics, minimizing the need for extensive dose-escalation studies.
AI-Driven Analysis of Clinical Trial Data
Traditional methods of analyzing clinical trial data often struggle to uncover subtle patterns or complex relationships within the data. AI algorithms, however, are exceptionally well-suited to this task. Their ability to handle high-dimensional data and identify non-linear relationships provides a deeper level of insight.
AI algorithms such as machine learning and deep learning can be applied to analyze various aspects of clinical trial data, including patient demographics, clinical outcomes, and biomarker measurements. These analyses can reveal hidden patterns and insights that would be difficult or impossible to detect using traditional statistical methods. For example, AI could identify subgroups of patients who respond differently to a treatment, or uncover unexpected correlations between certain biomarkers and treatment efficacy.
Successful applications of AI in clinical trial analysis include identifying predictive biomarkers for treatment response, optimizing treatment strategies based on individual patient characteristics, and accelerating the regulatory approval process for new drugs and therapies. For example, in oncology trials, AI has been successfully used to predict patient survival based on genomic and clinical data, allowing for more personalized treatment decisions.
AI in Biomarker Discovery and Validation
Biomarkers play a crucial role in monitoring disease progression and treatment response. AI significantly accelerates the discovery and validation of new biomarkers. This leads to better diagnostics and more effective treatments.
AI algorithms can analyze large datasets of genomic, proteomic, and clinical data to identify potential biomarkers that are associated with disease development, progression, or treatment response. These algorithms can also be used to validate the clinical utility of newly discovered biomarkers.
AI-Based Biomarker Discovery Methods
| Method | Data Type | Application | Advantages |
|---|---|---|---|
| Machine Learning (e.g., Support Vector Machines, Random Forests) | Genomic, Proteomic, Clinical | Identifying predictive biomarkers for disease risk, progression, or treatment response | High accuracy, ability to handle high-dimensional data |
| Deep Learning (e.g., Convolutional Neural Networks, Recurrent Neural Networks) | Imaging, Genomic, Proteomic | Identifying patterns in medical images, predicting treatment response from genomic data | Ability to learn complex patterns, high predictive power |
| Natural Language Processing (NLP) | Electronic Health Records (EHRs), Clinical Notes | Extracting relevant information from unstructured clinical data, identifying potential biomarkers | Automation of data extraction, ability to handle large volumes of unstructured data |
| Network Analysis | Genomic, Proteomic, Metabolic | Identifying key molecules and pathways involved in disease pathogenesis | Visualization of complex biological relationships, identification of potential drug targets |
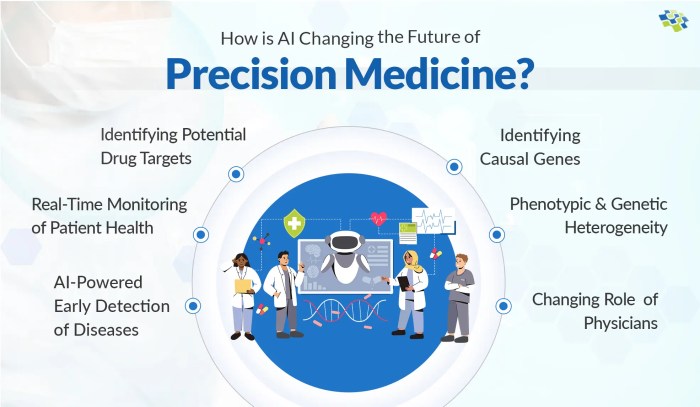
Closing Summary: The Role Of AI In Advancing Precision Medicine

Source: eularis.com
The integration of AI into precision medicine isn’t just a technological advancement; it’s a paradigm shift. While challenges remain, particularly in addressing ethical concerns and ensuring equitable access, the potential benefits are undeniable. The future of healthcare is undeniably intertwined with AI, promising a future where treatments are more effective, diseases are detected earlier, and patients receive care that’s truly tailored to their individual needs. The journey is just beginning, but the destination – a healthier future powered by intelligent technology – is within reach.
